NEWS: ALL UPDATES ON EU REGULATIONS AND GUIDELINES FOR FREE
RESPONSIBILITIES
In order to establish clear responsibilities, each cosmetic product should be linked to a Responsible Person established within the Community. The responsibilities of the Responsible Person are:
- Retention, UPDATING, and revision of the PIF
- Designation of the Safety Assessor
- Realisation of the product according to European Good Manufacturing Practice (ISO 22716:2007)
- Performing the notification operations to the EU Commission before introduction of the products on the market
- Management and organisation of the Cosmetic Survey for the report of possible undesirable effects to the Health Authorities of the Member State where the serious undesirable effect occurred
- Labelling layout and compliance with health regulations
- Sustainability of the product claims
REQUIREMENTS
In order to guarantee the compliance of every single product RP places on the market, he should be in possess (or he has to be assisted by an expert that is in possess) of the following skills:
- Technical
- Formulative
- Legal/Regulatory
- Manufacturing
- Claim Sustainability
- Quality System/Management
Thanks to our consolidated experience gained in the market and to our constant interaction with the Trade Associations and Health Authorities, Angel Consulting can offer you all the pertinent information or assume the role of the European Responsible Person.
REGULATION (EC) 1223/2009 – ART. 25
1) Without prejudice to paragraph 4, competent authorities shall require the responsible person to take all appropriate measures, including corrective actions bringing the cosmetic product into conformity, the withdrawal of the product from the market or its recall, within an expressly mentioned time limit, commensurate with the nature of the risk, where there is non-compliance with any of the following:
(a) the good manufacturing practice referred to in Article 8;
(b) the safety assessment referred to in Article 10;
(c) the requirements for the PIF referred to in Article 11;
(d) the provisions on sampling and analysis referred to in Article 12;
(e) the notification requirements referred to in Articles 13 and 16;
(f) the restrictions for substances referred to in Articles 14, 15 and 17;
(g) the animal testing requirements referred to in Article 18;
(h) the labelling requirements referred to in Article 19(1), (2), (5) and (6);
(i) the requirements related to product claims set out in Article 20;
(j) the access to information for the public referred to in Article 21;
(k) the communication of serious undesirable effects referred to in Article 23;
(l) the information requirements on substances referred to in Article 24.
2) Where applicable, a competent authority shall inform the competent authority of the Member State in which the responsible person is established of the measures which it has required the responsible person to take.
3) The responsible person shall ensure that the measures referred to in paragraph 1 are taken in respect of all the products concerned which are made available on the market throughout the Community.
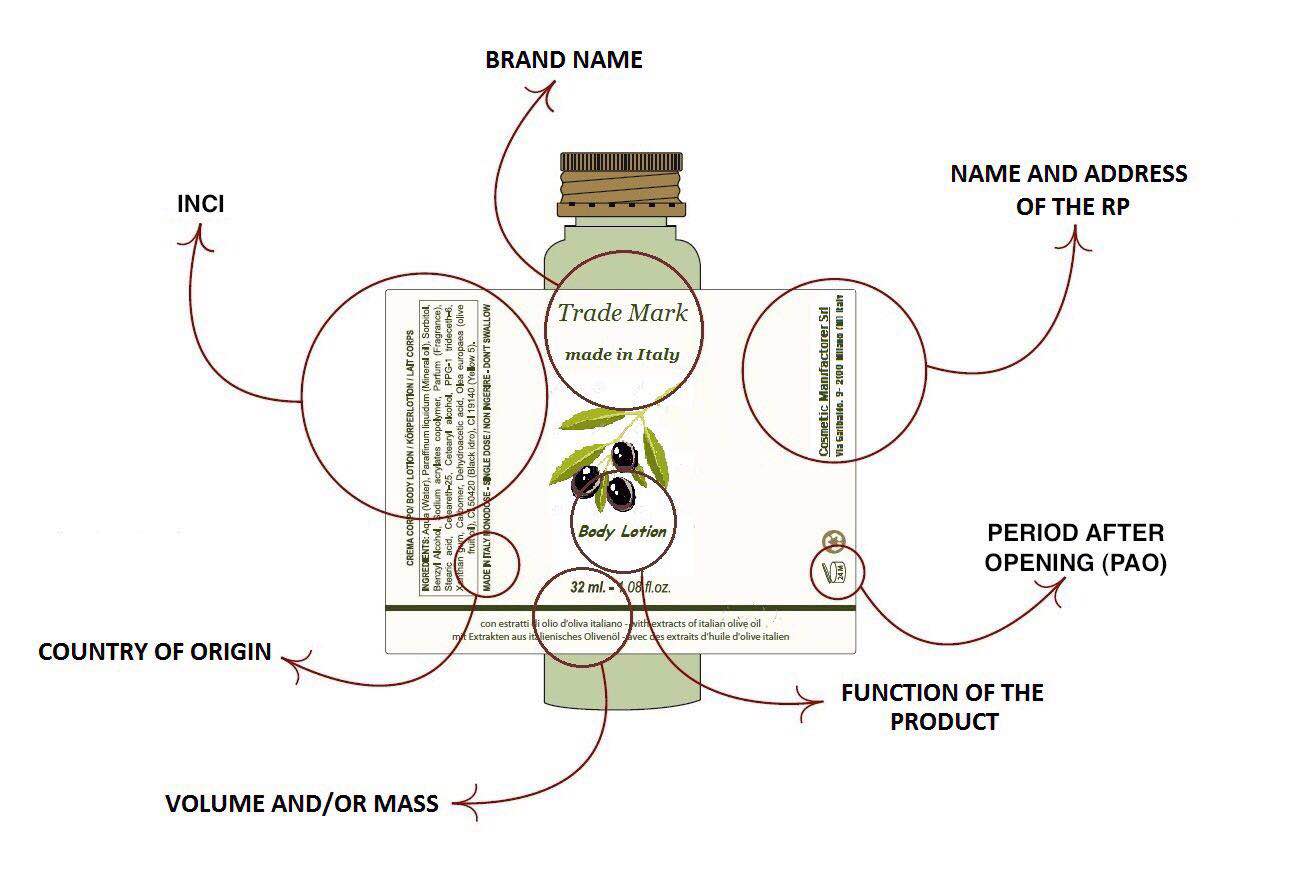
ABOUT LABELLING
The name and the address of the Responsible Person should be indicated on the label
Comments are closed.







